Introduction:
With progressive aging of the worldwide population, the number of newly diagnosed multiple myeloma (NDMM) cases are anticipated to escalate. Disease management for elderly, frail, and transplant-ineligible patients still remains a challenge. With our systematic review, we plan to summarize the available therapeutic options for frail, elderly, and transplant-ineligible NDMM patients.
Methods:
A comprehensive literature search was performed on May 27, 2020 on PubMed, Cochrane Library, and ClinicalTrials.gov. The search was not limited to any geographical area, date, or language. The keywords used in the search were: newly diagnosed, multiple myeloma, frail, elderly, transplant-ineligible, and treatment outcome. After subsequent screening, following the PRISMA guidelines, 24 ongoing/completed studies (n= 10,095) were included in the review.
Results:
Two and three drug combination regimens:
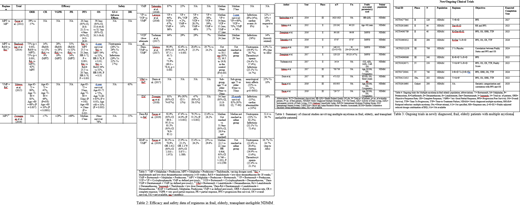
Among the two and three drug combinations (see tables 1 and 2), lenalidomide (R) and dexamethasone (d) (Rd) combination (n=1445) and bortezomib (V), melphalan (M) and prednisone (P) (VMP) combination (n=1059) have been the most extensively studied combinations thus far. In a phase 2 trial, Takezako et al. (2016) used 9 cycles of VMP followed by bortezomib maintenance exclusively in frail patients, which yielded an ORR of 87%, CR rate of 25%, and PFS of 36 months. The addition of bortezomib (V) to Rd (VRd) was also studied in a phase 3 (SWOG S0777) trial by Durie et al. (2017) (n=471) in transplant-ineligible patients, which showed superior therapeutic response rates (PFS 43 months, OS of 75 months, and ORR of 82%) in the VRd group compared to Rd (PFS of 30 months, OS of 64 months and ORR of 72%) alone. However, the three-drug combination of daratumumab (Dara)+Rd has overall shown the best treatment results (ORR 92.9%, CR 47.6% and VGPR 31.8%) as evidenced by Facon et. al (2019) (n=368). Although, when compared to Rd, Dara-Rd was associated with more neutropenia (50% versus 35.3%); however, Dara-Rd had an overall lower incidence of infections (32.1% versus 73.4%) and hence, lower discontinuation rate (32.4% versus 56.7%) when compared to Rd, respectively.
Four or five drug combination regimens:
Four and five drug combinations have been studied in two phase II/III trials (n=933) in transplant-ineligible patients. A phase two trial (Mateos, 2015) studying VMPRd in frail patients resulted in an ORR of 68%, CR 20%, and PFS of 25 months after 35 days of VMPRd induction, followed by dose de-escalation to twice monthly bortezomib-only maintenance until disease progression. Dara-VMP was compared with VMP alone in a phase 3 trial (Mateos, 2017) (n=700), in which 9 cycles of Dara-VMP (42 days each) were de-escalated to daratumumab-only maintenance. Thisshowed superior results in Dara-VMP (ORR 90.9% versus 73.9%, CR 42.6% versus 24.4%, and PFS was not reached in Dara-VMP group versus 18.1 months) compared to the VMP group. Moreover, this dose de-escalation also seemed to improve treatment tolerability, as the discontinuation rate due to adverse effects was also lower (4.9%).
Currently, various trials are ongoing to explore the connection between frailty status and associated treatment outcomes (table 3).
Conclusion:
Combination regimens, including agents like daratumumab and bortezomib, have exhibited promising results both in terms of superior efficacy and reduced adverse effects profiles. In addition, dose de-escalated maintenance therapies following induction therapies based on multidrug combinations have exhibited noteworthy success, with comparable efficacy and reduced toxicity rates in frail and transplant-ineligible NDMM patients. However, there remains an opportunity to further tailor the dose and type of drug combinations on a 'frailty-adjusted' basis, with trials designed around this risk stratification providing the highest quality data.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal